The Hydration of Elite Phase in Portland Cement
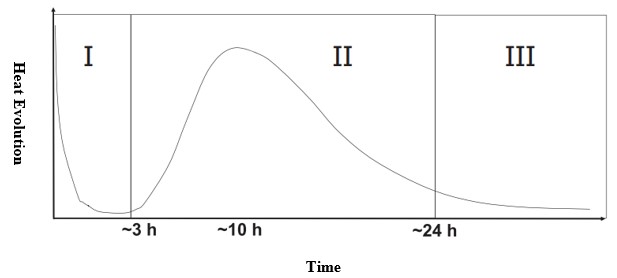
The dominant mechanisms of the hydration of Calcium Silicate (C3S) are generally discussed through the heat evolution curve. Although the curve is divided into seven areas in most resources, it is important to only discuss three parts of it (Figure.1): I- from the beginning to the end of the induction period, II- from the induction period to the end of the main peak of the hydration and III- the hydration after the main peak.
There are two main assumptions for the induction period of the Elite Phase hydration:
- Protective Membrane Theory
This is one of the oldest theories about hydration that is considered an acceptable justification for this process. The calculation of enthalpy indicates that the rate of the hydration process reduces in the induction period despite the high solubility of the Elite. According to this contradiction, some researchers suggested the formation of a low-stability calcium silicate hydrate (C-S-H) layer on the surface of the reacting particles as an obstacle to the reactivity. Therefore, unstable products determine the rate of dissolution. The obstacle layer for hydration is destroyed by assuming the conversion of these unstable products to stable products at the end of the induction period.
- Controlling dissolution by unsaturated condition
The dissolution rate of pure C3S is reduced non-linearly by reducing the saturation level of the solution e.g. when the system reaches equilibrium. To carry out the tests, the solutions were diluted sufficiently to prevent the precipitation of hydrates. Therefore, the obtained rates for the reaction do not necessarily indicate the real dissolution rates that occurred in the cement paste while hydration in which the hydrates almost precipitate at the same time. It is very complicated to measure such rates in real situations since it is hard to experimentally determine or separate the “free” surface of C3S being dissolved.
In real hydration, the dissolution and precipitation of the hydrates are accomplished simultaneously at the same rate (Since the solution is not able to store a significant amount of dissolved substances). The existence of hydrates on the surface of the reactants can disturb the dissolution process.
The surface topography is another difference between the pure dissolution tests and real hydration. The morphology of the observed voids in the pure dissolution test is too shallow and lenticular while the observed voids on the particles of C3S during the hydration are generally too deep and irregular. This indicates that the dissolution rates obtained from the pure dissolution tests may not reach the dissolution rate in hydration conditions directly and it is still necessary to make some efforts to explain these differences. In fact, the hydrated surface of C3S is adjacent to a solution that evolves during the time and therefore passes through different dissolution regimes that lead to a time-dependent topography. The dissolution tests quantitatively show that the dissolution rates are significantly reduced by the evolution of the system to equilibrium.
The simulation of the two theories of inactive layer and zone deactivation:
Boolard and Flet tried to find ways to differentiate between the Inactive Layer Theory (i.e. protective membrane theory) and the zone deactivation assumption (i.e. Dissolution Theory) in a set of stimulation tests. The results showed that both theories can lead to stimulations that reproduce conventional experimental properties such as thermal evolution and evolution of pore solution composition. However, the simulations based on the two different theories showed very different kinetics by evaluating the preventive effect of the Portlandite precipitation on hydration kinetics (by considering other things the same). There is a strong delay in the kinetics of the dissolution theory however, no delay is observed for the protective membrane theory. This is justified by the dissolution theory by explaining that there is no Calcium Hydroxide ion due to the lack of Calcium Hydroxide precipitation, and the composition of the solution in an unsaturated condition with C3S will remain in a small amount. Moreover, the dissolution rate of the protective membrane depends on reforming an unstable C-S-H to a more stable C-S-H. Therefore, preventing the precipitation of the Portlandite will not affect its reform and no delay is observed. Boolard and Flet suggested that identifying an concrete admixture that selectively prevents the precipitation of the Portlandite while not influencing the C3S and C-S-H can firmly differentiate the two theories.
Finally, the idea that a reaction is limited by a protective membrane as an obstacle for propagation must be abandoned. Therefore, the assumption of dissolution theory as a mechanism that is responsible for the induction period will remain valid.
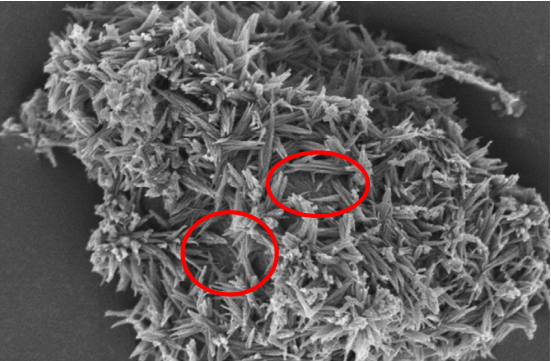
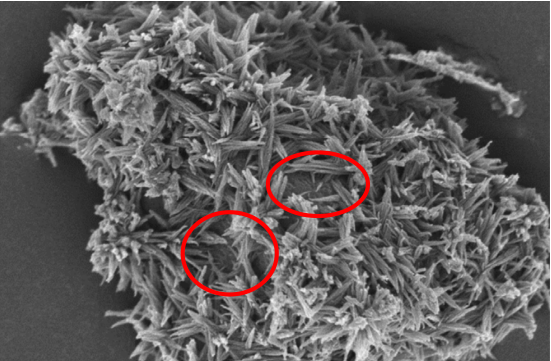
The Nano-Spherical morphology was observed for this hydration product in the early ages of the hydrated cement during the C-S-H artificial sediment studies recently. This product can be the unstable product formed within the first few minutes of the C3S hydration reaction. Koomar et al. reported the amorphous structure of this product by carrying out the X-ray diffraction test after making the C-S-H artificial sediment in a condition of pH=11. The Ca/Si ratio was less than 1.25 in this product and did not imitate the structure of the silicate chain “dreierketten” generally found for C-S-H.
The C-S-H growth on Elite, cement, or filler particles can have a foil appearance, but it generally seems that the foils are merged to form the ordinary sharp or needle-shaped morphology. The C-S-H growth is still based on the layer structure of Tobermorite with respect to the quasi-one-dimensional growth of the needles.
The second stage: The main peak of the hydration
Regarding the discussion about the dominant mechanisms on the main peak of the hydration, it is important to note that fitting this curve does not confirm the model used to produce the curve. Also, it is necessary to evaluate the hydration models based on their physical, chemical, and microstructure compatibility.
The diffusion and impact layer were two conventional assumptions to explain the peak of the thermal evolution in the literature. These assumptions are not able to explain the experimental observations. In the following, two newer assumptions including limited growth and dissolution limitation are introduced and discussed.
The diffusion layer assumption
The diffusion layer theory assumes that the C-S-H precipitation on the cement particles, a thickening layer is formed which is thickened as much as it acts as an obstacle for diffusion. Several models depend on this theory before 2000.